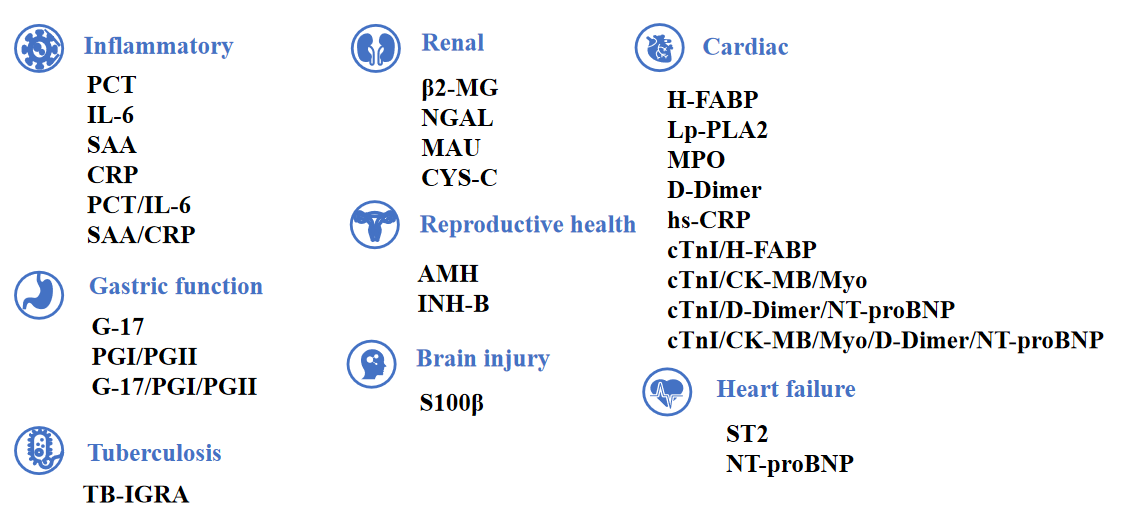
Growth-stimulating expression gene 2 protein (ST2)
The kit is used to quantitatively determine the content of soluble growth-stimulating expression gene 2 protein (ST2) in human serum or plasma.
Rapid detection of single reagent (15 minutes)
Convenient storage and transportation at room temperature
DESCRIPTION
Successfully obtained in the EU CE access certification
Passed ISO13485 certification
ST2: a novel biomarker for heart failure
ST2 is a member of the interleukin-I(IL-1)receptor family. When the heart continues to be under pressure, the expression of ST2 increases, which indirectly promotes myocardial hypertrophy and myocardial fibrosis, affects cardiac function, and greatly increases the readmission and mortality of patients with heart failure.
ST2 is released when cardiomyocytes stretch, neutralizing its ligand IL-33 (1, 2). It is also associated with inflammation during the MI and HF (3). As a decoy receptor of IL-33 receptor (ST2L), an appropriate amount of sST2 can prevent uncontrolled inflammation. However, redundant sST2 could also block the advantageous biological effect of IL-33 due to its competitive role against ST2L, which will eventually cause HF (3). Recently, sST2 is frequently reported to be associated with CVDs, especially HF (5–6). The ST2 pathway in CVD is shown in Figure 1.
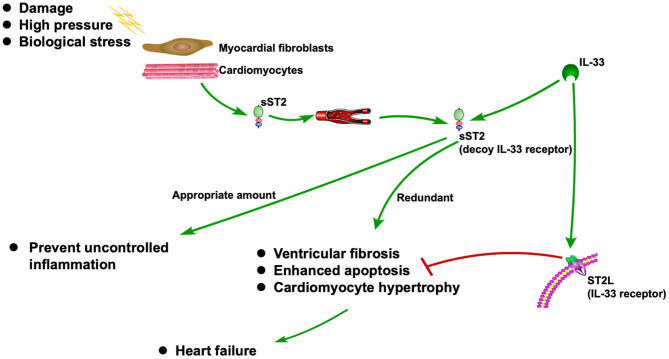
Figure 1
ST2 pathway in CVD
Biomarkers have made a significant contribution to the diagnosis and prognosis of disease. Although strongly associated with inflammatory and autoimmune diseases, ST2 has also been found to play a role in the diagnosis and prognosis of CVD (4, 7). As one of the isoforms of ST2, sST2 has recently become a promising prognostic indicator for patients diagnosed with HF and a useful tool for risk stratification (8). Due to its prognostic value, sST2 was recommended by the American College of Cardiology Foundation (ACC)/American Heart Association (AHA) as an important biomarker for monitoring HF patients in 2013 (9).
ST2 yielded strong, independent predictive value for all-cause and cardiovascular mortality, and HF hospitalization in chronic HF, and deserves consideration to be part of a multimarker panel together with NT-proBNP and hs-TnT.
Clinical significance:
• Auxiliary diagnosis of heart failure
• For monitoring the efficacy of heart failure
• For risk stratification and prognostic risk assessment of heart failure
Applicable departments:
Emergency department, cardiac surgery, cardiology, chest pain center, Clinical Laboratory, ICU, CCU.
References:
1. Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, et al. Circulation. (2002)
3. Marino R, Magrini L, Orsini F, Russo V, Cardelli P, Salerno G, et al. Ann Lab Med. (2017)
4. Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, et al. Am J Respir Crit Care Med. (2001)
5. Dudek M, Kałuzna-Oleksy M, Migaj J, Straburzyńska-Migaj E. Adv Clin Exp Med. (2020)
6. McCarthy CP, Januzzi JL, Jr. Soluble ST2 in Heart Failure. Heart Fail Clin. (2018)
SPECIFICATION
| Sample type | serum, plasma |
| Report time | 15min |
| Storage | 4-30℃, sealed and kept away from light and dry, the validity period is 18 months |
| Specifications | 25 tests/box;50 tests/box |

 Request
Request
Applicable Instrument

CATALOGS
 2 pages
2 pages